Page 19 - CIJ May 2025- Digital Edition
P. 19
COVER STORY
3. Where should regulatory
focus lie in pharma and
cleanroom environments?
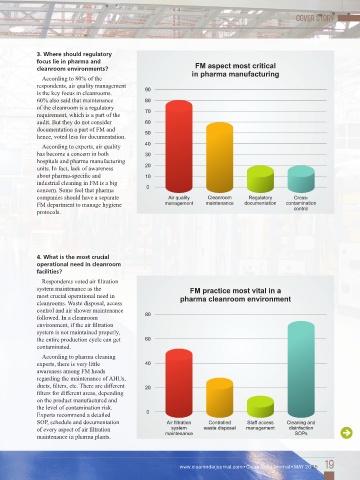
According to 80% of the
respondents, air quality management
is the key focus in cleanrooms.
60% also said that maintenance
of the cleanroom is a regulatory
requirement, which is a part of the
audit. But they do not consider
documentation a part of FM and
hence, voted less for documentation.
According to experts, air quality
has become a concern in both
hospitals and pharma manufacturing
units. In fact, lack of awareness
about pharma-specific and
industrial cleaning in FM is a big
concern. Some feel that pharma
companies should have a separate
FM department to manage hygiene
protocols.
4. What is the most crucial
operational need in cleanroom
facilities?
Respondents voted air filtration
system maintenance as the
most crucial operational need in
cleanrooms. Waste disposal, access
control and air shower maintenance
followed. In a cleanroom
environment, if the air filtration
system is not maintained properly,
the entire production cycle can get
contaminated.
According to pharma cleaning
experts, there is very little
awareness among FM heads
regarding the maintenance of AHUs,
ducts, filters, etc. There are different
filters for different areas, depending
on the product manufactured and
the level of contamination risk.
Experts recommend a detailed
SOP, schedule and documentation
of every aspect of air filtration
maintenance in pharma plants.
19
www.cleanindiajournal.com•Clean India Journal•MAY 2025